Experiment and simulation on oxidation characteristics of natural gas
-
摘要:
在流动管反应器中对压力为0.1 MPa、温度范围为500~1 850 K、当量比分别为0.5、1.0与3.5的工况条件下天然气(90%甲烷/7%乙烷/3%丙烷,体积分数)的氧化过程进行了实验测试。同时,通过全局敏感性分析方法,构建了天然气的简化反应动力学机理(38组分和149反应),并对天然气的氧化特性进行了数值计算。结果表明:随着当量比增大,燃料发生氧化反应的起始温度与终止温度逐渐升高,CO生成与消耗完全对应的反应温度逐渐升高,NO的生成量逐渐降低。天然气的简化反应机理可以很好地预测天然气氧化过程中主要组分摩尔分数随温度变化的整体趋势;但是在C3H8、C2H2、NO、NO2的起始反应温度或摩尔分数峰值的预测上与相应实验值存在差异。
Abstract:The oxidation of natural gas (90% methane/7% ethane/3% propane,volume fraction) was experimentally tested in the flow reactor under the conditions of the pressure of 0.1 MPa,the temperature range of 550-1 850 K,the equivalence ratios of 0.5,1.0 and 3.5.Meanwhile,through the global sensitivity analysis,the reduced reaction kinetic mechanism of the natural gas (including 38 species and 149 reactions) was established,and the oxidation characteristics of the natural gas were simulated.The results showed that,with the increase of equivalent ratio,the starting and ending temperatures of fuel oxidation reaction gradually rose,the reaction temperature of CO generation and complete consumption raised,and the production of NO reduced.The reduced reaction mechanism of the natural gas had a good prediction of the variational trends of the mole fractions of the main species with temperature during the oxidation of the natural gas.However,there existed still some deviations in the prediction of the starting reaction temperature or the peak mole fractions of some species,such as C3H8,C2H2,NO and NO2.
-
表 1 实验工况及气体组成
Table 1. Experiment conditions and gas compositions
当量比 摩尔分数 CH4 C2H6 C3H8 O2 N2 He 0.5 0.040 5 0.003 15 0.001 35 0.197 550 0.743 183 0.014 267 1.0 0.040 5 0.003 15 0.001 35 0.098 775 0.371 592 0.484 633 3.5 0.040 5 0.003 15 0.001 35 0.028 221 0.106 169 0.820 610 -
[1] BULAT G,JONES W P,MARQUIS A J.NO and CO formation in an industrial gas⁃turbine combustion chamber using LES with the Eulerian sub⁃grid PDF method[J].Combustion and Flame,2014,161(7):1804⁃1825. [2] ALMEIDA D S,LACAVA P T.Analysis of pollutant emissions in double⁃stage swirl chamber for gas turbine application[J].Energy Procedia,2015,66:117⁃120. [3] LYRA S,CANT R S.Analysis of high pressure premixed flames using equivalent reactor networks for predicting NOx emissions[J].Fuel,2013,107(9):261⁃268. [4] STARIK A M,KOZLOV V E,LEBEDEV A B,et al.Application of reactor net models for the simulation of gas⁃turbine combustor emissions[J].International Journal of Sustainable Aviation,2014,1(1):43⁃57. [5] MOHAMED H,TICHA H B,MOHAMED S.Simulation of pollutant emissions from a gas⁃turbine combustor[J].Combustion Science and Technology,2004,176(5/6):819⁃834. [6] FICHET V,KANNICHE M,PLION P,et al.A reactor network model for predicting NOx emissions in gas turbines[J].Fuel,2010,89(9):2202⁃2210. [7] JARPALA R,BURLE N A,VOLETI M,et al.Effect of swirl on the flame dynamics and pollutant emissions in an ultra⁃lean non⁃premixed model gas turbine burner[J].Combustion Science and Technology,2017,189(10):1832⁃1848. [8] 尉曙明,索建秦.航空衍生工业燃气轮机双燃料贫燃预混低污染燃烧技术[J].航空动力学报,2015,30(9):2049⁃2057.WEI Shuming,SUO Jianqin.Aero⁃derivative industrial gas turbine dual fuel lean premixed low emission combustion technology[J].Journal of Aerospace Power,2015,30(9):2049⁃2057.(in Chinese) [9] ABIAN M,GIMENEZ⁃LOPEZ J,BILBAO R,et al.Effect of different concentration levels of CO2 and H2O on the oxidation of CO:experiments and modeling[J].Proceedings of the Combustion Institute,2011,33(1):317⁃323. [10] ABIAN M,ALZUETA M U,GLARBORG P.Formation of NO from N2/O2 mixtures in a flow reactor:toward an accurate prediction of thermal NO[J].International Journal of Chemical Kinetics,2015,47(8):518⁃531. [11] BURKE S M,METCALFE W,HERBINET O,et al.An experimental and modeling study of propene oxidation:Part 1 speciation measurements in jet⁃stirred and flow reactors[J].Combustion and Flame,2014,161(11):2765⁃2784. [12] GAO Z H,HU E J,XU Z H,et al.Low to intermediate temperature oxidation studies of dimethoxymethane/n‑heptane blends in a jet‑stirred reactor[J].Combustion and Flame,2019,207:20⁃35. [13] BURKE U,METCALFE W K,BURKE S M,et al.A detailed chemical kinetic modeling,ignition delay time and jet⁃stirred reactor study of methanol oxidation[J].Combustion and Flame,2016,165:125⁃136 [14] 高振华.二甲氧基甲烷/正庚烷混合燃料的宽温度范围实验与反应动力学模型研究[D].西安:西安交通大学,2020.GAO Zhenhua.Experimental and kinetic modeling study of dimethoxymethane/n‑heptane blends in wide temperature range[D].Xi'an:Xi'an Jiaotong University,2020.(in Chinese) [15] 郑玮琳,庞历瑶,张世杰,等.组分变化对甲烷氧化特性影响[J].航空动力学报,2021,36(1):15⁃24.ZHENG Weilin,PANG Liyao,ZHANG Shijie,et al.Effect of composition variance on mechane oxidation characteristics[J].Journal of Aerospace Power,2021,36(1):15⁃24.(in Chinese) [16] 殷阁媛.2,4,4⁃三甲基⁃1⁃戊烯基础燃烧特性和化学反应动力学机理研究[D].西安:西安交通大学,2019.YIN Geyuan.Comprehensive chemical kinetic modeling study on 2,4,4⁃trimethyl⁃1⁃pentene combustion[D].Xi'an Jiaotong University,2019.(in Chinese) [17] LIU Y X,YU D,TIAN D X,et al.An experimental and modeling study of oxidation of 1,2,4⁃trimethylcyclohexane with JSR[J].Proceedings of the Combustion Institute,2019,37(1):437⁃444. [18] CURRAN H J,GAFFURI P,PITZ W J,et al.A comprehensive modeling study of n‑heptane oxidation[J].Combustion and Flame,1998,114(1/2):149⁃177. [19] BAKALI A E,DAGAUT P,PILLIER L,et al.Experimental and modeling study of the oxidation of natural gas in a premixed flame,shock tube,and jet⁃stirred reactor[J].Combustion and Flame,2004,137(1):109⁃128. [20] HUNTER T B,LITZINGER T A,WANG H,et al.Ethane oxidation at elevated pressures in the intermediate temperature regime:experiments and modeling[J].Combustion and Flame,1996,104(4):505⁃523. [21] METCALFE W K,BURKE S M,AHMED S S,et al.A hierarchical and comparative kinetic modeling study of C1⁃C2 hydrocarbon and oxygenated fuels[J].International Journal of Chemical Kinetics,2013,45(10):638⁃675. [22] KEROMNES A,METCALFE W K,HEUFER K A,et al.An experimental and detailed chemical kinetic modeling study of hydrogen and syngas mixture oxidation at elevated pressures[J].Combustion and Flame,2013,160(6):995⁃1011. [23] ZHOU C W,LI Y,O'CONNOR E,et al.A comprehensive experimental and modeling study of isobutene oxidation[J].Combustion and Flame,2016,167:353⁃379. [24] LI Y,ZHOU C W,SOMERS K P,et al.The oxidation of 2⁃butene:a high pressure ignition delay,kinetic modeling study and reactivity comparison with isobutene and 1⁃butene[J].Proceedings of the Combustion Institute,2017,36(1):403⁃411. [25] ZHOU C W,LI Y,BURKE U,et al.An experimental and chemical kinetic modeling study of 1,3⁃butadiene combustion:Ignition delay time and laminar flame speed measurements[J].Combustion and Flame,2018,197:423⁃438. [26] MORRIS M D.Factorial sampling plans for preliminary computational experiments[J].Technometrics,1991,33(2):161⁃174. -
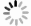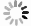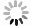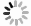







 下载:
下载: